[ Quality ]

At Graviti Pharmaceuticals, quality is not just a requirement—it is our foundation. We are committed to manufacturing pharmaceuticals that meet the highest global regulatory standards, ensuring safety, efficacy, and reliability in every product.
Our quality-driven culture is embedded in every stage of our operations, from raw material selection to final product release, guided by a zero-defect mindset and a continuous improvement approach.
We don’t just meet standards—we set them.

[ Audit Success ]

Quality isn’t just a benchmark for us; it’s the core of everything we do. Our recent regulatory inspection in 2023 resulted in zero 483 observations, reinforcing our unwavering commitment to compliance, operational excellence, and global best practices. This achievement reflects our dedication to producing high-quality pharmaceuticals that meet the most stringent regulatory requirements.

[ Quality by Design ]

We follow the Quality by Design (QbD) approach, ensuring that every process is meticulously crafted to build quality into the product from the ground up.
Our science-based, data-driven quality approach allows us to consistently deliver world-class pharmaceuticals.

[ Quality Policy ]
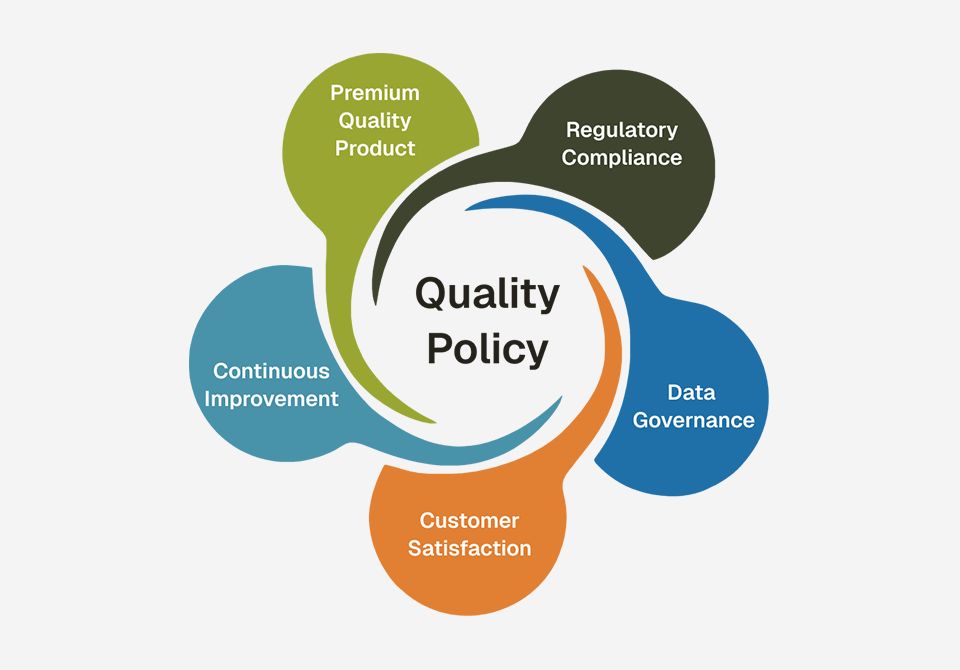
Graviti is committed to providing safe, effective and reliable pharmaceuticals by adhering to the highest quality standards and global regulatory requirements.
To meet this commitment, Graviti fosters a quality-focused culture that lays emphasis on premium quality products, regulatory compliance, data governance, customer satisfaction and continuous improvement.

[ Data Integrity Policy ]
Data integrity is at the heart of regulatory compliance and patient safety. At Graviti Pharmaceuticals, we ensure end-to-end data governance, protecting the reliability and accuracy of our records.
By eliminating data vulnerabilities and maintaining real-time records, we reinforce transparency, reliability, and trust in our pharmaceutical processes.

[ Technology Enhanced Compliance ]

Nucleus is our dedicated Quality & Compliance Hub, designed to integrate cutting-edge analytical technology, regulatory intelligence, and compliance expertise.
Nucleus is Graviti’s Quality & Compliance Hub, uniting advanced analytics, regulatory intelligence, and compliance expertise. Our mobile-first, paperless platform ensures data integrity and streamlined operations, with integrated digital systems across functions. Ongoing automation and data acquisition enhancements further drive precision, efficiency, and intelligence

[ Safety Policy ]

Safety is paramount for our workforce, our products and our environment.
Through rigorous safety audits, training, and preventive measures, we ensure a secure and compliant working environment.

[ Evolving Excellence ]
to excellence
Embedding quality systems into our company’s mission and long-term strategies to drive sustainable success.
to excellence
Establishing control procedures, allocating resources, and ensuring thorough documentation and communication for seamless execution.

to excellence
Developing robust systems that offer clear guidance and enable ongoing evaluation to maintain excellence.

to excellence
Conducting internal audits and management reviews, followed by corrective and preventive actions to address gaps and drive improvement.